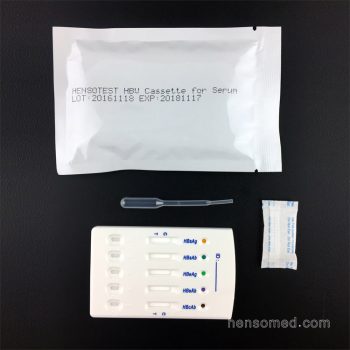
HBV 5 in 1 Combo Test Panel
- Format: Combo Card
- Test Item: HBsAg, HBsAb, HBeAg, HBeAb, HBcAb
- Specimen: Serum/Plasma/Whole Blood
- Size: 3.0mm, 4.0mm
- Accuracy: over 99.7%
- Results read in 10-15 minutes
- Shelf time: 2 years
- Description
- Instruction for Use
- Ordering Information
- Inquiry
HBV 5 in 1 Combo Test Panel is a rapid, qualitative immunoassay for the determination of hepatitis B markers (HBsAg, HBsAb, HBeAg, HBeAb, HBcAb) in human serum or plasma, as an aid in the clinical assessment of hepatitis B infection. Both testing and result interpretation are intended to be used by medical professionals only. The test should not be used without appropriate supervision.
Features
- Fast read in 10-15 minutes
- Accurate over 99.7%
- Use easily and cost effectively
- Visually interpreted
Packaging
Box of 15T, Ctn of 600T
Operating instruction for Diagnostic kit for HBV Infection Marker
(surface antigen, surface antibody, core antibody, e antigen, e antibody) (Colloidal Gold)
【Product Name】
The common name: Diagnostic kit for HBV Infection Marker (surface antigen, surface antibody, core antibody, e antigen, e antibody) (Colloidal Gold)
【Packing Specification】Card Type
【Intended use】
Qualitative detection of the five hepatitis B markers HBsAg, HBsAb, HBeAg, HBeAb, HBcAb in human serum (or plasma), for clinical diagnosis.
【Detection Principle】
The product uses the colloidal gold and membrane chromatography technology, and measures HBsAg, HbeAg in serum with dual-antibody sandwich method, and measures HbsAb with dual-antigen sandwich method, and measures HbeAb and HBcAb with neutralization competitive inhibition method.
【Major component ingredients】
- Hepatitis B two pairs of semi- test card
- A copy of operating instruction
【Sample requirements】
- Collect venous blood samples in sterile conditions, and separate serum or plasma as soon as possible to avoid hemolysis.
- When testing, you should make full use of fresh sample. If the sample can not be detected in time, it can be refrigerated for 3 days at 2-8 ℃. Long-term preservation needs to be frozen at -20 ℃, and it can’t be repeatedly freezing and thawing.
- The conventional anticoagulant does not affect the test results.
- The hemolysis, viscous and high-fat blood samples do not apply to this reagent.
- Adding 0.1% NaN3 in the samples does not affect the result.
【Test Method】
Please read the instructions for the test kit completely before carrying out test.
- Revert the test board and the testing samples to room temperature(20-30℃).
- The right side of the test board should be kept horizontal from the original package, from left to right, respectively corresponding to HBsAg, HBsAb, HBeAg, HBeAb, HBcAb. With a small straw to take subjects’ serum, and add into 5 sample wells of the test board by drops (70μl per well or two drops).
- Observe and record the experimental result within 15 minutes. Weakly positive samples appear test line in 15-20 minutes. Determination after 30 minutes is invalid.
Note: Take out test paper from the original packaging, and it should be used within 1 hour as soon as possible, especially at room temperature above 30 ℃ or in a highly humid environment.
【Results determination】
(1)HBsAg、HbsAb、HBeAg (Sandwich method)
Positive Negative Invalid
Negative: Only one purple bar (control line) in the control C zone.
Positive: Detecting T zone There are two purple bars in the control C zone.
Invalid: Detecting T zone There is no purple bar in the control C zone.
(2)HBeAb HBcAb (Competition method)
Positive Negative Invalid
Negative: Detecting T zone There are two purple bars in the control C zone.
Positive: Only one purple bar (control line) in the control C zone.
(Weakly positive sample may appear a very thin response line at the test line).
Invalid: Detecting T zone There is no purple bar in the control C zone.
【The interpretation of test results】
- The purple bands, which appear at the quality-control control lines, is used as the internal control standards of the test paper strip.
- The purple bands appear gradation phenomenon at the testing line, when testing different specimens, and this is due to high and low concentrations of surface antigen.
【The limitations of the testing methods】
This method is clinical auxiliary diagnostic product, any of the tested positive samples should be further confirmed by application of other methods (such as EIA).
【Performance indicators of products】
sAg≥2ng eAg≥2NCU sAb≥30mIU eAb≥4NCU cAb≥2NCU
【Instructions and Notes】
- This product is a disposable in vitro diagnostic products, and the same test card can not be reused.
- This product is a qualitative reagent screening the presence of hepatitis B markers, but it can not determine the surface antibody content of the samples.
- This product is suitable for testing human serum or plasma samples.
- Experimental environment should shelter from the wind and avoid making the experiment in too high temperature, high humidity or too dry conditions.
- Operation according to infectious disease laboratory test procedures.
- When a large number of samples tested, please mark well, to avoid confusion.
- Experiments confirm that hepatitis A, hepatitis C, HIV, syphilis, HEV, HGV do not interfere with the results.
- It should not be frozen or use after it is past due.
【Storage conditions and valid period】
Preserve at 2-30 ℃ in sealed dark dry place, and the valid period is 24 months.
【References】
- Control practical handbook for bio-products and their quality standards for raw and auxiliary materials, Volume III, national audio-visual publishing house, Miao Yong, Zang Guangzhou editor in chief.
- The preparation instructions for extrinsic diagnostic reagents guideline; State Food Drug Administration Device [2007] No. 240.
| REF. | Description | CBM/ctn | KGS/ctn |
| L113901 | HBV cassette 3.0mm for Serum, 5 in 1 Combo | 0.069 | 9 |
| L113902 | HBV cassette 3.0mm for Whole Blood , 5 in 1 Combo | 0.069 | 9 |